
CONVENIENT DOSING1
Strike with once-daily dosing for a full course of therapy in 17 drops1
Complete treatment course in one bottle1
One drop the day before cataract surgery begins to treat your patients’ postoperative inflammation and pain.1,2
ILEVRO® 0.3% Dosing Schedule1
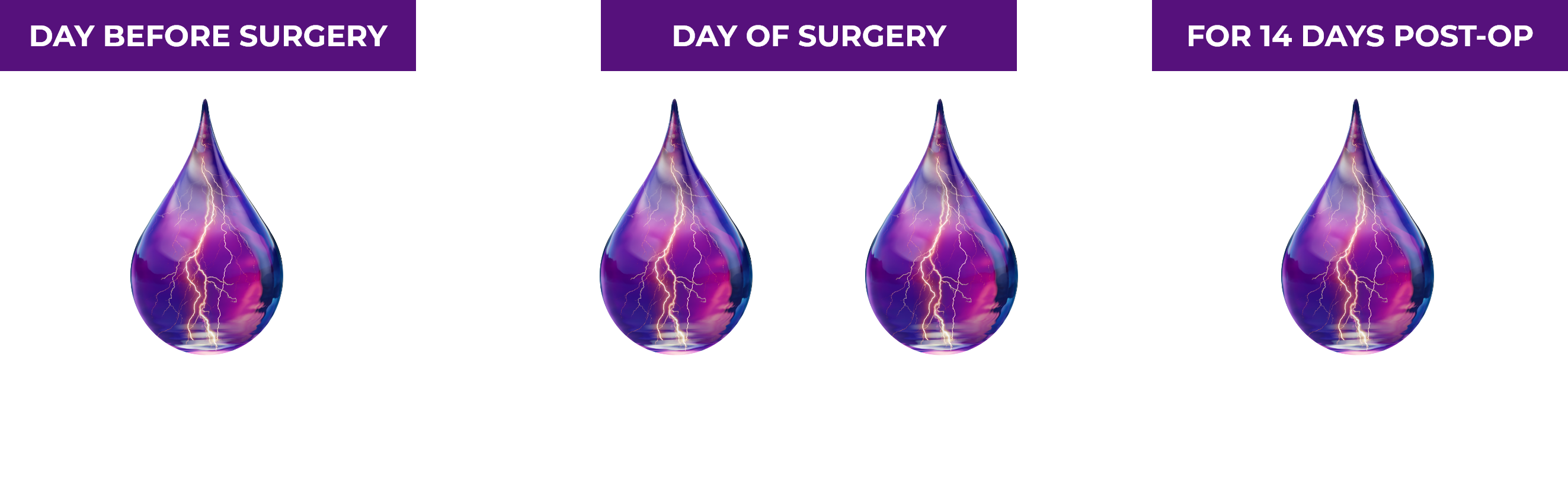
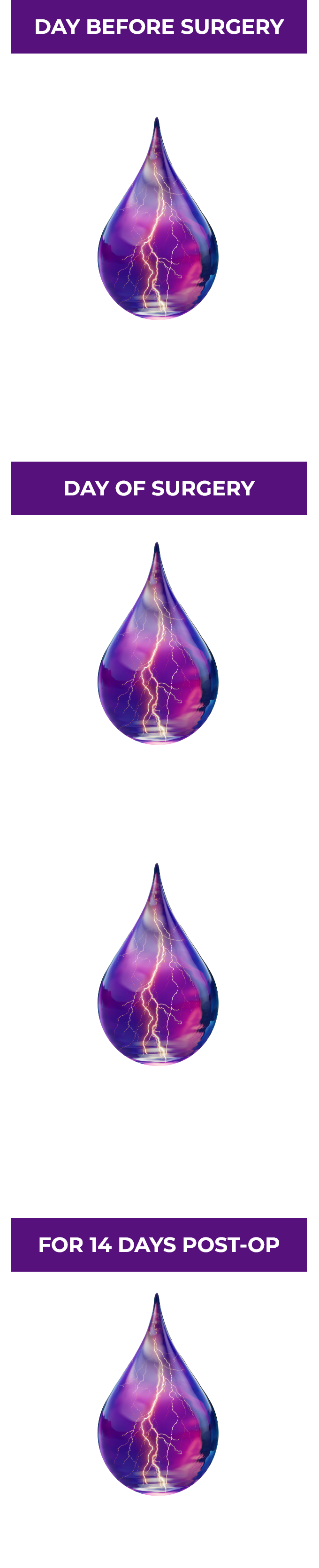
Once-daily dosing may increase patient compliance.2
The only prodrug NSAID formulated for once-daily postoperative use
Use more than 1 day prior to surgery or use beyond 14 days after surgery may increase patient risk and severity of corneal adverse events.1
There is no generic equivalent for ILEVRO®3
Designed for potency and penetration1
Your patients may be able to save on their script
Data provided through MMIT and are current as of 2/2024. The information provided in this website is not a guarantee of coverage or payment (partial or full). Actual benefits are determined by each plan administrator in accordance with its respective policy and procedures. Nothing herein may be construed as an endorsement, approval, recommendation, representation or warranty of any kind by any plan or insurer referenced herein.
References: 1. ILEVRO (nepafenac ophthalmic suspension) 0.3% [package insert]. Harrow IP, LLC; 2023. 2. Modi SS, Lehmann RP, Walters TR, et al. Once-daily nepafenac ophthalmic suspension 0.3% to prevent and treat ocular inflammation and pain after cataract surgery: phase 3 study. J Cataract Refract Surg. 2014;40(2):203-211. 3. Orange Book: Approved Drug Products With Therapeutic Equivalence Evaluations [database online]. Silver Spring, MD: US Food and Drug Administration; 2023. http://www.fda.gov/cder/ob/default.htm. Updated July 2023. Accessed August 8, 2023.