Proven efficacy for MANAGING inflammation and pain associated with cataract surgery1,2
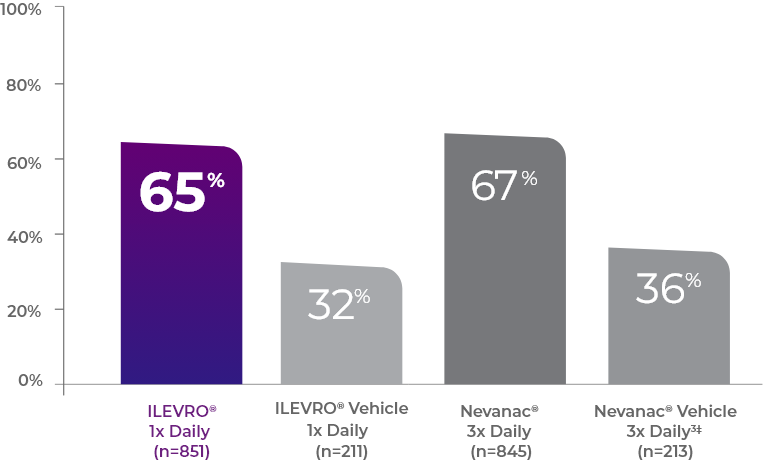
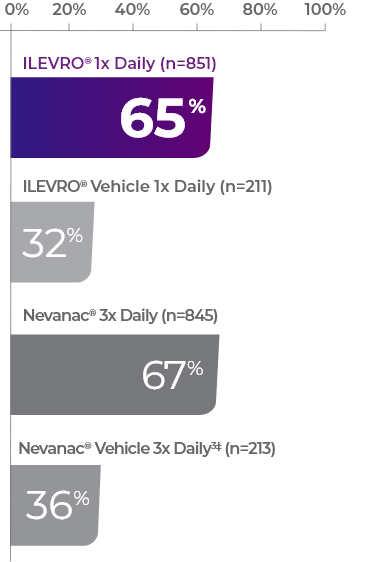
PERCENTAGE OF PATIENTS WITH INFLAMMATION COMPLETELY RESOLVED† AT DAY 141
The difference between ILEVRO (65%) and Nevanac (67%) was not statistically significant (p<0.0001%)
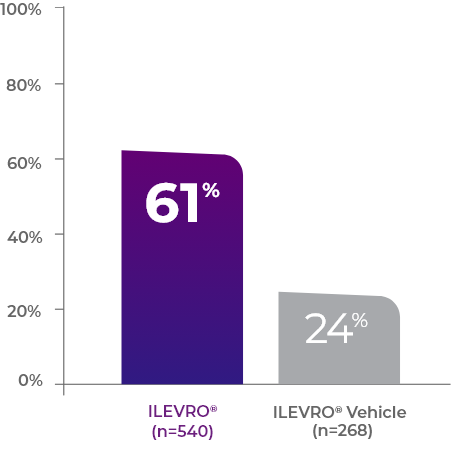
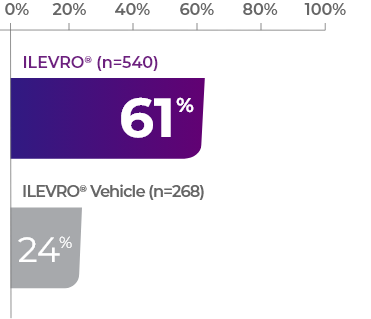
In both of these studies
as many ILEVRO® patients had complete resolution vs vehicle1
Use more than 1 day before surgery or use beyond 14 days after surgery may increase patient risk and severity of corneal adverse events.1

IN THESE STUDIES
Complete resolution† was defined as zero aqueous cells and zero flare.3
PAIN FREE AS EARLY AS DAY 11
Percentages of ILEVRO®-treated patients who experienced mild to no pain in both studies3,4
n=894
In both of these studies
of patients treated with ILEVRO® experienced mild to no pain at post-op day 13,4

IN THESE STUDIES
Pain free was defined as a score of zero on a pain scale from 0 to 5.2
Designed for potency and penetration1
Your patients may be able to save on their prescription
Data provided through MMIT and are current as of 2/2024. The information provided in this website is not a guarantee of coverage or payment (partial or full). Actual benefits are determined by each plan administrator in accordance with its respective policy and procedures. Nothing herein may be construed as an endorsement, approval, recommendation, representation or warranty of any kind by any plan or insurer referenced herein.
Defined as zero cells and zero flare on a scale of 0 (none) to 4 (>30 cells) and 0 (no visible flare) to 3 (severe), respectively.3
Nevanac® vehicle percentage calculated using the ITT population.3
References: 1. ILEVRO (nepafenac ophthalmic suspension) 0.3% [package insert]. Harrow IP, LLC; 2023. 2. Modi SS, Lehmann RP, Walters TR, et al. Once-daily nepafenac ophthalmic suspension 0.3% to prevent and treat ocular inflammation and pain after cataract surgery: phase 3 study. J Cataract Refract Surg. 2014;40(2):203-211. 3. Data on file. Clinical Overview. Harrow IP, LLC; 2023. 4. Data on file. Summary Review. Harrow IP, LLC; 2023.